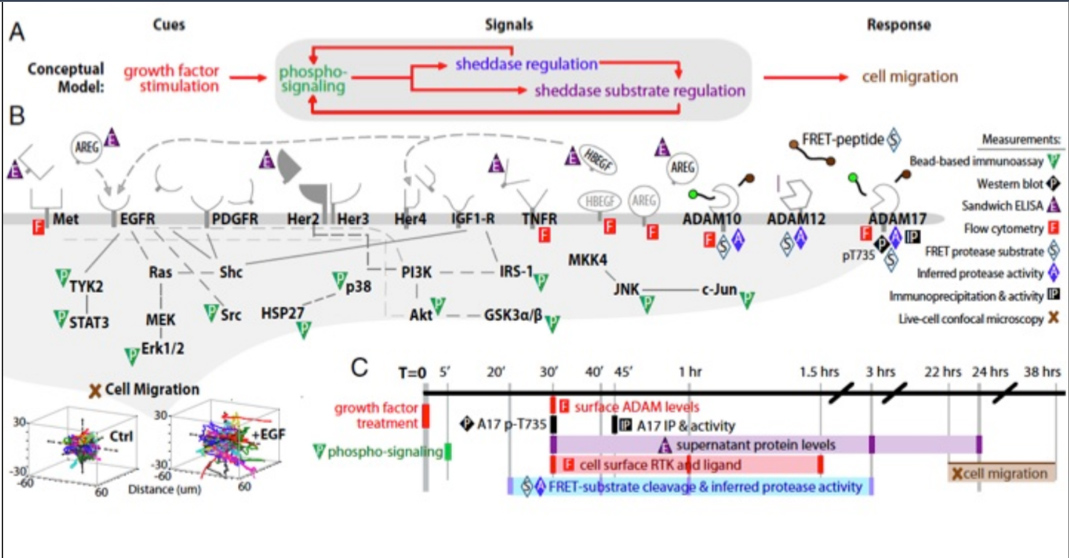
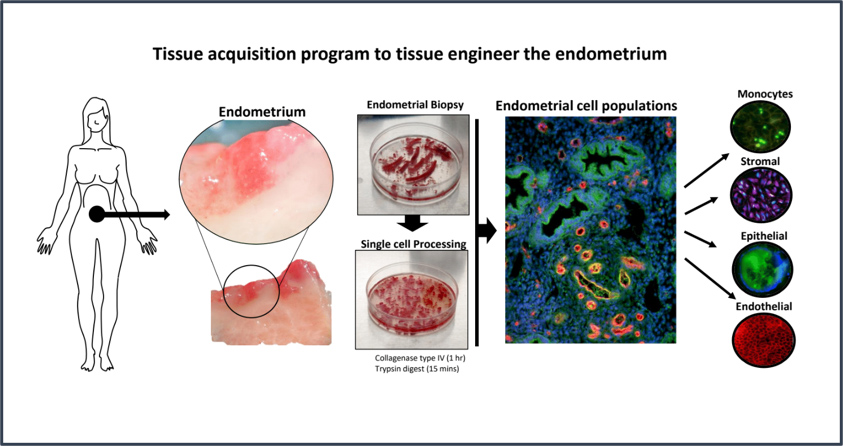
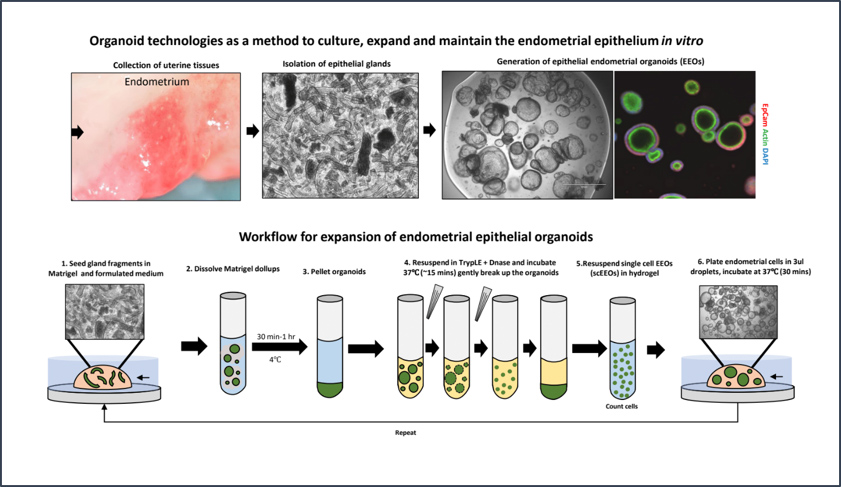
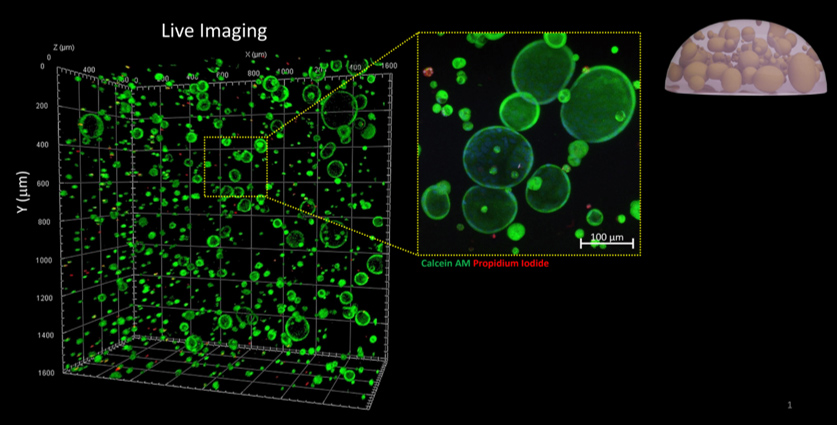
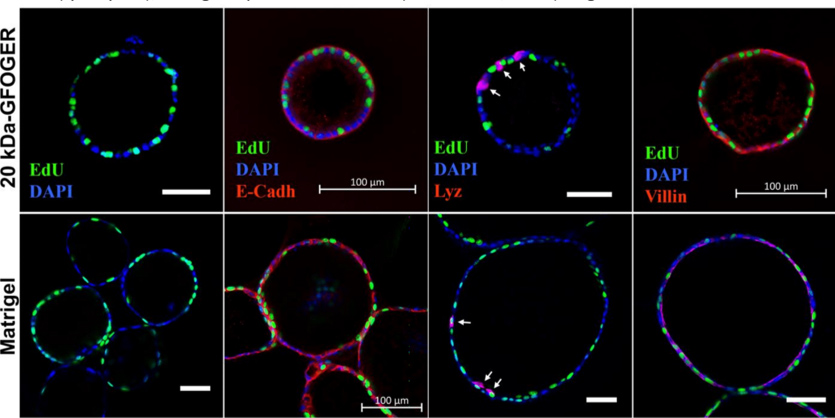
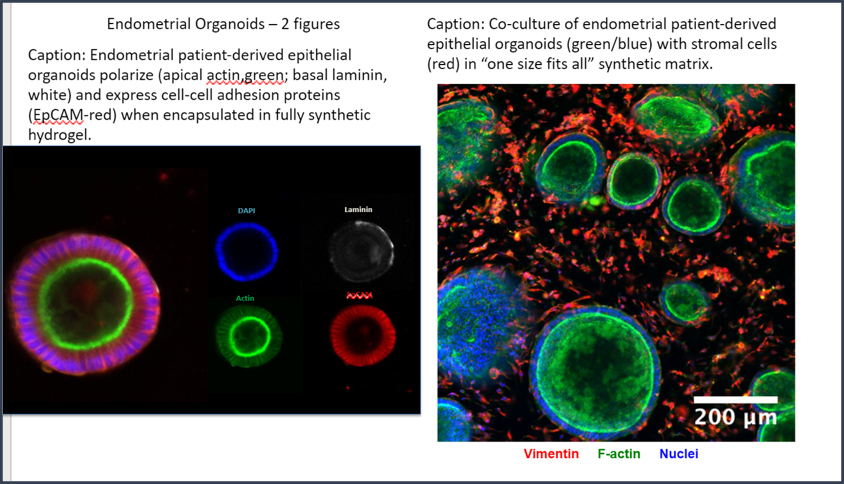
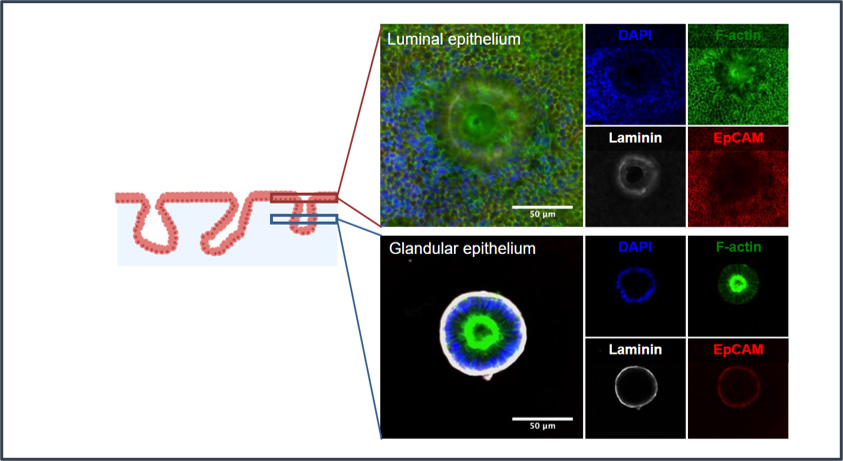
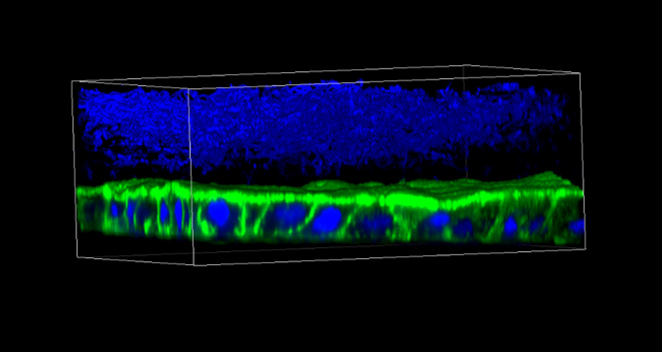
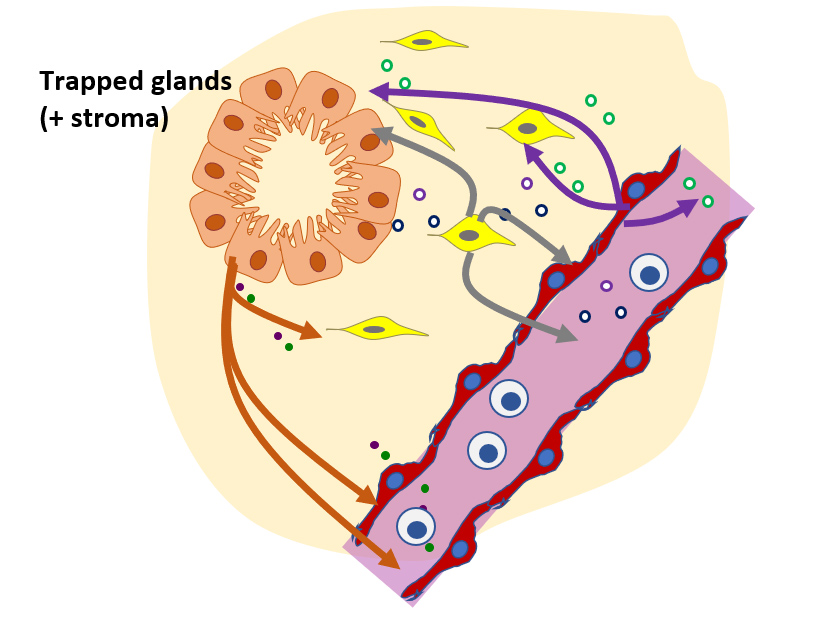
CGR’s “Systems Biology” Approach
The observations expressed in the questions above – and others regarding the huge disparity in patients’ ages are when they first discover symptoms, their differences in responses to therapies, and the wide range of co-morbidities – led us to bring a “systems biology” approach to the study of endometriosis, adenomyosis and related diseases.
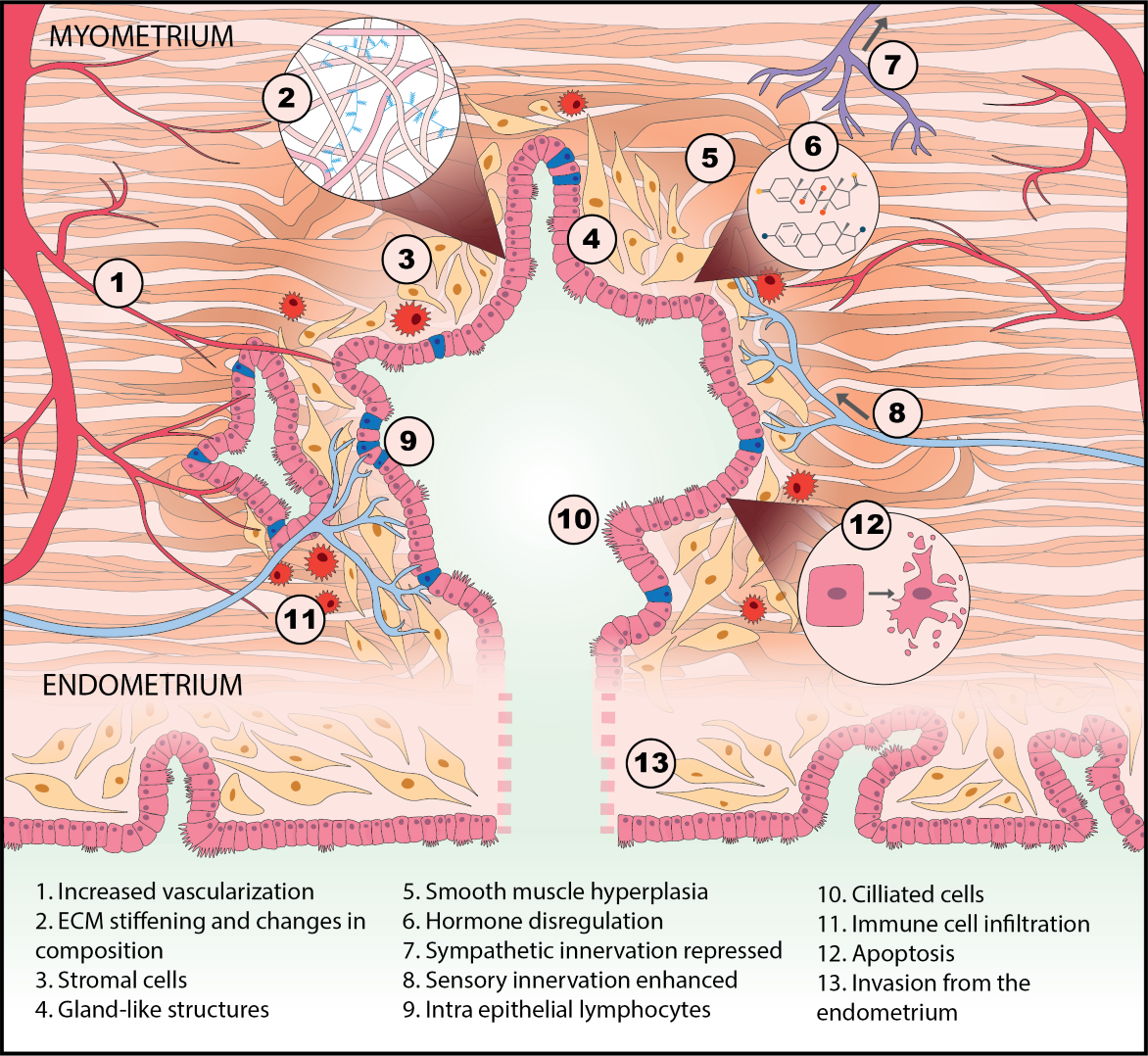
Endometriosis is currently staged by the numbers, size, and location of lesions and adhesions. Why isn’t endometriosis also characterized by molecular markers related to disease mechanism, prognosis, and treatment, like breast cancer? One reason is that cancers have “somatic mutations”—tumor cells have mutated genes that drive malignancy. The mutations in cancer patients can be identified, and in many cases, linked to prognosis and therapies. Endometriosis, like rheumatoid arthritis, Type 2 diabetes, Alzheimer’s, Crohn’s disease, and other chronic inflammatory diseases, is not known involve somatic mutations as a disease driver.
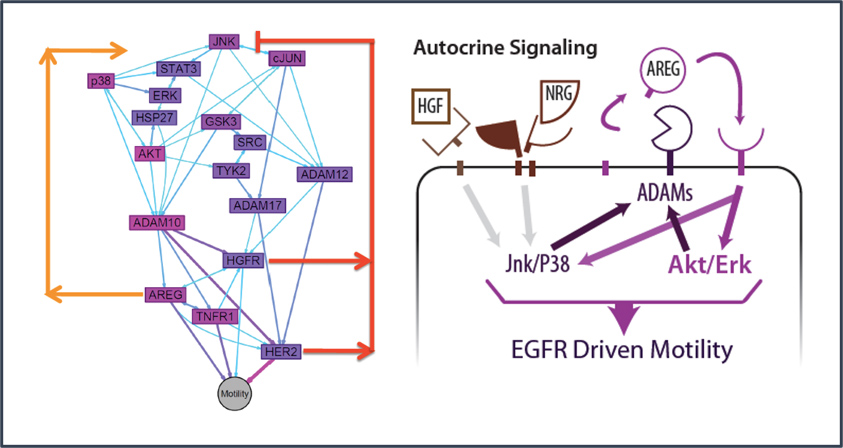
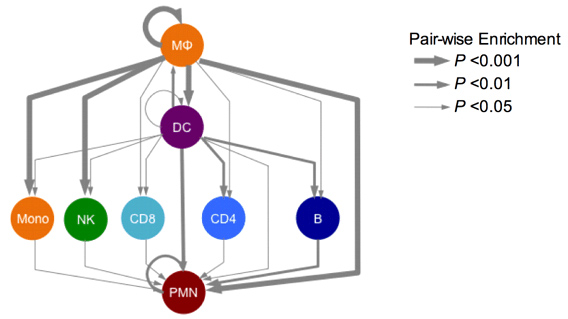
We borrowed from systems biology research in these other diseases to propose that irregularities in cell-cell communication networks – which might arise to many different kinds of gene-environment interactions – might provide insight into general mechanisms and even discern different patient groups. Although many researchers for decades have discovered how individual molecules or pathways are aberrant in endometriosis patients, in the systems approach, we examine how pathways operate in networks, and networks of different kinds intersect to control phenotype. This approach has led to identification of possible molecular subclasses of patients implicating new disease targets.